Page 16 - Delaware Medical Journal - January 2017
P. 16
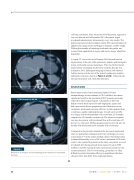
CT Abdomen 11/02/15
cell lung carcinoma. After discussion with the patient, aggressive care was desired and in December 2015, the patient began nivolumab (administered intravenously every two weeks). The patient experienced clinical improvement. She reported improved appetite and energy levels and began to maintain a stable weight. Within three months of initiating nivolumab, the patient was weaned from supplemental oxygen and was no longer wheelchair dependent.
A repeat CT scan at the end of January 2016 showed interval improvement in the size of the pulmonary nodules and the hepatic ����������������������������������������������������������������� improvement on imaging studies since systemic therapy was initiated in 2012. Subsequent imaging in March 2016 showed further decrease in the size of the patient’s pulmonary nodules and hepatic lesion (as shown in Figure 1 and 2). Clinically, she tolerated treatment well, with mild joint pain.
DISCUSSION
Based upon results from a randomized phase III trial, immunotherapy with nivolumab, a PD-1 inhibitor, has shown ���������������������������������������������������������������� exposed to other targeted agents. All patients in this trial
had previously been exposed to anti-angiogenic agents and
had experienced disease progression since their most recent treatments; nivolumab was more effective in these patients than everolimus, another indicated therapy. Overall survival (OS) was longer with nivolumab than with everolimus, 25.0 months compared to 19.6 months, respectively. The objective response rate was also greater with nivolumab than with everolimus (25 percent vs. 5 percent). Median progression-free survival was 4.6 months with nivolumab and 4.4 months with everolimus.
Compared to the patients included in the previously mentioned trial, our patient had exhausted more lines of therapy (6 versus
a maximum of 3 in the study) and had a poorer functional status (estimated Karnofsky score of 50 versus a minimum of 70 in the study). Also, unlike the patients in the study, our patient received nivolumab after having already been exposed to an m-TOR inhibitor, and after having already experienced central nervous system metastasis. This is worth noting, given that it is often ����������������������������������������������������������������� characteristics that differ from study populations.
CT Abdomen 03/24/16
FIGURE 2
Reduction in hepatic lesion following three months of nivolumab therapy.
16
Del Med J | January 2017 | Vol. 89 | No. 1